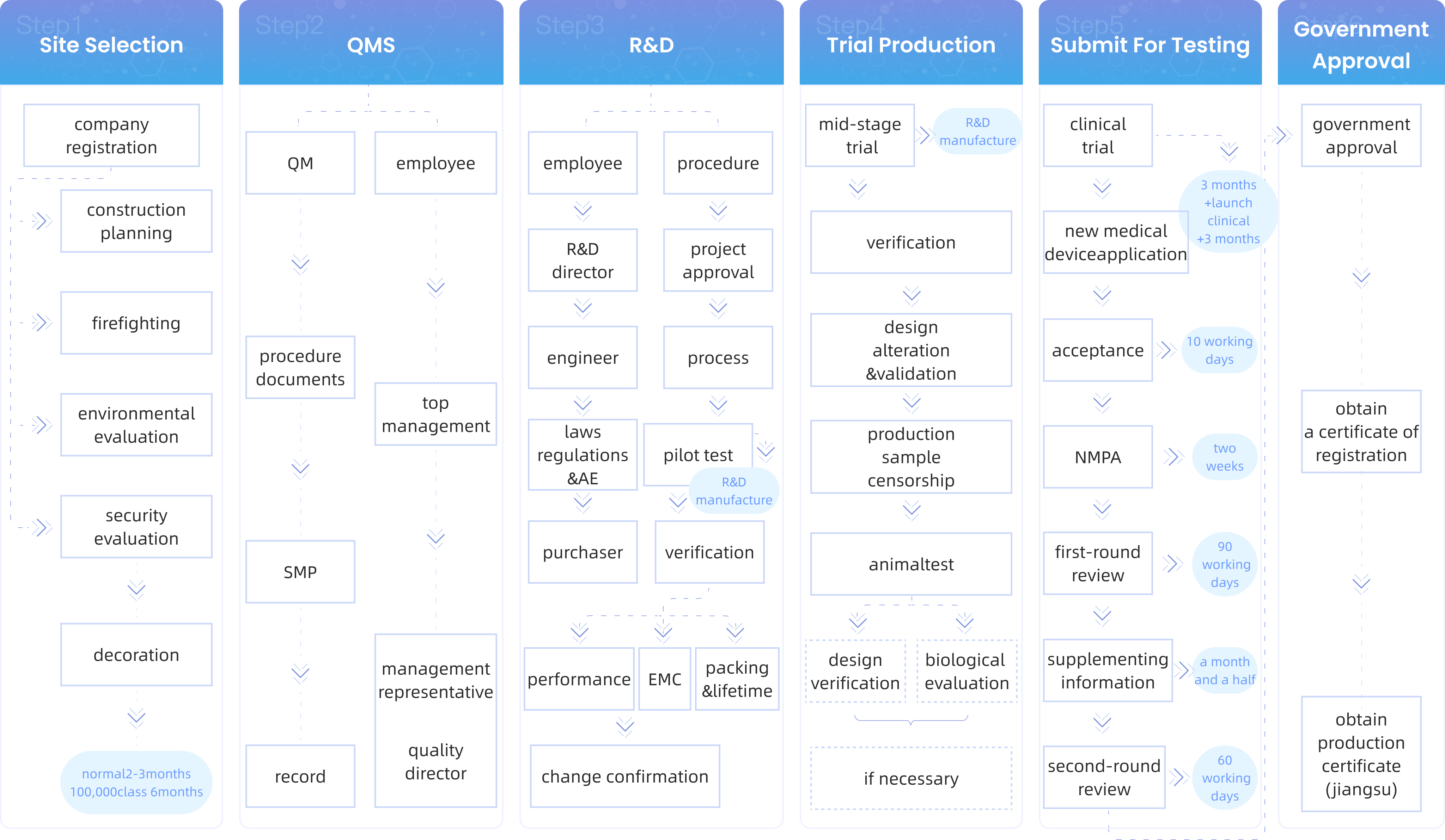
Under the Marketing Authorization Holder (MAH) system, overseas projects no longer need to invest a substantial amount of capital in building compliant manufacturing facilities and organizing production. For overseas projects, it is only necessary to establish a domestic joint venture company, and all subsequent production issues can be entrusted to the CDMO platform for end-to-end services.
This approach allows for a focus on research and development, strengthening localization efforts, and enhancing core competitiveness.
It avoids issues such as heavy capital investment, equipment idle time, and the substantial expenses associated with personnel costs and overall operational expenses.
Through a professional CDMO service platform, the time required to obtain product registration certificates is further reduced.
Once the MAH obtains the certification, they can quickly establish sufficient production capacity with the assistance of the CDMO service platform, rapidly penetrate the market, and meet societal needs.